Inadequate dosimetry or description of experimental design has been a common shortcoming of many radiofrequency bioeffect experiments performed in the past. Without adequate dosimetric definition, experiments cannot be replicated nor can the information gained from the experiment be used to define the expected consequences of equivalent human exposures.
To facilitate compilation of the minimum required data, researchers at USAFSAM, Brooks Air Force Base, Texas, developed an RFR Bioeffects Research Data Sheet (Figure 7.1). The form indicates what information should be recorded, and all the information is very important to those desiring to replicate an experiment or evaluate it. The required data are divided into four basic areas of concern as follows:
1. Description and condition of experimental data. Date and time of exposure are important for evaluating seasonal and diurnal effects. Provide a
Figure 7.1.
A data sheet for RFR bioeffects
research.
detailed description of the experimental subject, including the type of animals and their source. if biological substances are being investigated, note the source and method of preparation. A physical description of the experimental subject--including mass, length, width, and breadth--is the minimum data needed for theoretical evaluation of the SAR expected in the subject. These data are particularly important in the case of in vitro exposures such as cell suspensions and isolated tissues. In such cases define both the size of the biologic material and the nature of the surrounding suspension. if nonperturbing temperature probes are available, monitor and record the temperature of the experimental subject during exposure; if not, use available standard temperature-monitoring devices to measure the temperature of the subject prior to and immediately after exposure.
2. Environmental conditions. Environmental conditions can affect the subject's temperature-regulation capabilities and response to radiation. Measure and report the mean, minimum, and maximum values. Note any unusual environmental factors such as extreme lighting conditions, presence of noxious fumes, and use of hypobaric or hyperbaric conditions. Take care not to place environment-recording instruments in the RF fields; this can lead to inaccurate readings as well as perturbation of the exposure fields.
3. Definition of RFR exposure, including localized SAR. Define the RFR exposure conditions with as much precision as possible. Polarization can be defined as in Section 3.3.5. In the case of an unrestrained animal, note whether the orientation is random or preferred. "Polarization of the field" means whether the field is linearly, circularly, or elliptically polarized. Record the directions of the K and E vectors. If a frequency spectrum analyzer is available, record the width of the exposure frequency band and any anomalies introduced by pulse characteristics. measure and record the drift of the generator output, and report average and extremes. Determine the uniformity of the exposure field by mapping the field in the volume to be occupied by the subject; always do this without the subject in the field but with the subject-holding device in the same position as during exposure. Measure the incident fields by methods such as the dipole antenna, loop antenna, standard-gain horn, and broad-band isotropic RF monitor. Methods for measuring SARs may include calorimetry, localized temperature measurement,differential power techniques, or thermographic measurement in cadavers or models. Rate the condition of the subject (restrained, unrestrained, anesthetized, or unanesthetized).
4. Equivalence of animal exposure to that expected for man under similar exposure conditions. Determine the equivalent human-exposure frequency by finding the equivalent point on the SAR-versus-frequency curve (see Section 6.1) or determine an approximate equivalent from the following equation (see Chapter 2):
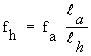 (Equation 7.1)
(Equation 7.1)where
![]() = equivalent human exposure frequency
= equivalent human exposure frequency
![]() = animal exposure frequency
= animal exposure frequency
![]() = length of animal
= length of animal
![]() = length of human
= length of human
Then use![]() to determine the average SAR expected in man
at this frequency by reading the SAR for the appropriate
polarization from the appropriate figure in Section 6.1.
Determine the equivalent exposure by
to determine the average SAR expected in man
at this frequency by reading the SAR for the appropriate
polarization from the appropriate figure in Section 6.1.
Determine the equivalent exposure by
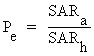 (Equation 7.2)
(Equation 7.2)where
![]() = equivalent human exposure (mW/cm²)
= equivalent human exposure (mW/cm²)
![]() = average SAR in exposed animal (W/kg)
= average SAR in exposed animal (W/kg)
![]() = average SAR in human expected at equivalent
frequency (W/kg per mW/cm²)
= average SAR in human expected at equivalent
frequency (W/kg per mW/cm²)
7.2.2. Holding Devices for Experimental Subjects
Because RF fields interact with dielectric materials, select with care the holding devices for experimental subjects. A variety of materials in common use include Lucite, Plexiglas, glass, Teflon, and a number of foam materials. Avoid all electrical conductors because they cause major perturbations in RF fields. The magnitude of the perturbation produced by subject-holding devices varies with the exposure frequency, electrical properties, mass, geometry, and orientation of the material. At 30 MHz a massive Lucite cage causes only minor perturbations of the fields, while at 10 GHz the same cage causes extreme field attenuation and perturbation of the fields. Glass rods and sheets of Lucite cause major perturbations in the 1- to 10-GHz frequency band if the materials are aligned in the same direction as the E-field; however, if these materials are aligned for minimum E-field aperture, the field perturbations will be minimal. SAR measurements have shown that dielectric interfaces can cause large deviations in localized SAR in tissue cultures exposed in test tubes. This is particularly true at the hemispherical surface at the bottom of a standard test tube for certain frequencies. Avoid potential problems with tissue cultures by properly orienting the test tube (less perturbation is observed for H polarization than E polarization) or by circulating or agitating the exposed material.
Experimenters at USAFSAM have found that rodent holders constructed from a rectangular section of Lucite, with numerous perforations for ventilation and with blocks of low-loss foam material filling the two open ends, can be used for planewave fields where the field is incident on the blocks of foam material. This device provides animal restraint with minimal field perturbation and minimal stress on the animal.
Since experimental conditions, availability of materials, and experimental objectives vary widely, detailed discussion of subject-holding devices is not appropriate. Experimenters should select the best materials available, test far-field perturbation at the frequencies of interest, and use only materials found to cause minimum perturbation. The quantity of dielectric material must be kept to a minimum, as should the use of electrical conductors. If materials that perturb the fields are used, they should not be aligned with the E vector. When the device is completed, measurements should be made to quantify the amount of field perturbation that it causes.
7.2.3. Exposure Devices
The commonly used planewave and near-field exposure devices are briefly reviewed in this section.
Planewave Exposure Devices--Anechoic chambers are the most used exposure devices. With an appropriate antenna system, these chambers provide versatile exposure conditions for bioeffects experiments. At lower frequencies, however, the size becomes prohibitive and the cost is generally high.
The TEM-mode chamber offers the advantages of being versatile in size, relatively inexpensive, and relatively broad-banded. It can be instrumented to measure SAR continuously with only three power meters, incident-power density is easily measured, and polarization effects can be measured. Since the TEM-mode chamber is particularly suitable for lower frequency exposures, it also complements the anechoic chamber. At higher frequencies (above 500 MHz), however, the size of the TEM-mode chamber must be significantly smaller to ensure that higher-order modes are cut off, which limits the usable exposure space. On the other hand, at these higher frequencies the anechoic chamber is generally reasonable in size and affordable in price.
When constructing a TEM chamber, pay special attention to the tapered transitions to ensure good impedance matches. Impedance mismatches cause standing waves in the chamber that may not be detected by power meters at the input and output ports. You can detect mismatches either by measuring timedomain reflection or by mapping the E-and H-fields inside the chamber. In every case, map the fields inside the chamber to ensure single-mode operation.
When higher-order modes and standing waves are not present, the E-field strength between the plates in the chamber can be found from
and
where
V = the rms voltage between the center conductor and the outer conductor of the line
P = the input power in watts
![]() = the characteristic impedance of the chamber
= the characteristic impedance of the chamber
The equivalent incident planewave power density can be found from
with the E-field in rms V/m.
Other planewave exposure devices include the circular waveguide and cell culture irradiator. Circular-waveguide exposure devices are relatively inexpensive and small in size, and they can be instrumented to constantly monitor the SAR in the exposed animal. These devices are power efficient and can provide almost continuous exposure of animals over long periods of time. This system is also desirable for chronic exposures of a large number of animals; however, incident-power density in the waveguide is difficult to measure. Five stable power meters are required to measure SAR; present designs limit the size of the experimental subject to that of a large rat, and the circular polarization of the field limits the advantage of being able to determine polarization effects. This is also a narrow-band device, and present models operate at 918 and 2450 MHz.
Cell-culture irradiators have the advantages of low cost, small size, and extremely good power efficiency; but they suffer from narrow-band operation, lack of field-measuring capability, and high gradient fields. To eliminate effects of hot spots with these devices, be sure to circulate the cellculture fluid.
Near-Field Exposure Devices--Near-field exposure devices basically provide E- and H-fields that can be controlled independently in phase and magnitude at the position of the exposed model so that any field impedance condition can be simulated. The following is a brief description of some common exposure devices.
One type of near-field exposure device is a resonant
cavity. Guy et al. (1974b) designed a resonant cavity for
![]() and
and ![]() mode resonance at 144 MHz. Each mode is fed by
a separate probe, with variable control of the relative phase and amplitude of the power
delivered to the feeds. The exposed subject is typically oriented
in the center of the cavity, at the position of maximum E-field and zero
H-field for the
mode resonance at 144 MHz. Each mode is fed by
a separate probe, with variable control of the relative phase and amplitude of the power
delivered to the feeds. The exposed subject is typically oriented
in the center of the cavity, at the position of maximum E-field and zero
H-field for the ![]() mode and maximum H-field and
zero E-field for the
mode and maximum H-field and
zero E-field for the ![]() mode. The resultant E- and
H-fields are, therefore, in space quadrature and
independently controllable in phase and magnitude at the
position of the exposed model so that any field impedance
condition can be simulated.
mode. The resultant E- and
H-fields are, therefore, in space quadrature and
independently controllable in phase and magnitude at the
position of the exposed model so that any field impedance
condition can be simulated.
Another exposure device is the near-field synthesizer developed by the National Bureau of Standards (NBS) (Greene, 1974); this device also can produce fields of several combinations. It consists of two parallel plates that produce a uniformly distributed E-field between them, and a loop inductor between the plates can provide an H-field parallel to the axis of the loop. The loop can also be rotated over any angle with respect to the plate, so the relative orientation between E- and H-fields can be varied from parallel to perpendicular.
An interesting comparison between the dosimetric results on scaled phantoms obtained using the resonant cavity and the near-field synthesizer is described by Guy et al. (1976a).
Anechoic chambers also can be used for the near-field exposures of phantoms and experimental animals (Iskander et al., 1978, 1981). Using different radiation sources and varying the distance between the source and the exposed subject allows a large variety of near-field exposures to be obtained. For simple sources (e.g., electric dipoles and loop antennas), the E- and H-fields at the location of the subject can be calculated. In general, however, particularly for more complicated sources, measuring these fields with suitable E- and H-field probes is advantageous.
7.2.4. Incident-Field Measurements
The procedure for measuring the RF incident fields at the location of the subject basically depends on the exposure conditions. For planewave or far-field exposures, apart from polarization and direction of propagation, only the incident-power density has to be measured. The planewave power density can be determined from the measured magnitude of the E- or H-field. In the near field, however, the E- and H-fields are not necessarily in phase or related by a constant wave impedance. Therefore, the magnitude and the direction of each E- and H-field must be measured independently at several points in the exposure region.
Depending on the type of detector, two basic techniques are used formonitoring RFR levels: (1) devices that measure power by sensing a temperature change due to energy absorption (thermocouple types) and (2) devices that use diodes to produce a current or voltage related to the electromagnetic energy. The thermocouple type of device has the advantage of accurate measurement in pulsed fields and a linear response with the incident-power density. The readout is accomplished by RF heating, however, so the instrument has a slow response and is susceptible to drift with environmental temperature, particularly in the lower ranges. The diode detector devices have the advantage of being extremely sensitive; they also have the major disadvantage of a limited dynamic range caused by the diodes' nonlinear response.
For planewave incident-power density measurements, several instruments are available, such as General Microwave, Holaday, Narda, and NBS EDM series. With all of these an orthogonal antenna system is used, and the antenna outputs are summed to provide a readout related to total incident power density. Take care when choosing an instrument to assure that appropriate range and pulse characteristics are known and that the instrument will respond to these field parameters. In making these measurements, measure the fields over the volume to be occupied by the experimental subject, first in empty space and then with the subject-holding device in place. This ensures that the effect of the subject-holding device is negligible. When using a single antenna, manually rotate it to measure each of the three orthogonal components of the field; then square each of these three components and add them to obtain the square of the total field. For linear polarization, first rotatd the antenna to the position of a maximum reading; then take the other two orthogonal measurements from that reference. This will usually result in the first reading being considerably larger than the other two, thus decreasing errors caused by inexact antenna positioning for the second and third measurements.
7.2.5. Measurement of Specific Absorption Rates
The SAR measurement is very useful in dosimetry. In cases where nonuniform exposures occur or where incident-power density cannot be measured, SAR is the only measurement that allows definition of the RF exposures. There are five basic techniques for measuring the SAR in biological systems and phantoms:
1. Differential power measured in a closed exposure system
2. Rate of temperature change measured with noninterfering probes
3. Calorimetric techniques
4. Thermographic techniques
5. Implantable E-field probes
The latter four methods are also suitable for near-field SAR measurements.
Differential-Power Technique--For the differential-power technique, use directional couplers and power meters on all input and output ports of the exposure device. In the case of waveguide and TEM-mode exposure devices, monitor the input, output, and reflected powers and determine the absorbed power by
where
![]() = power (watts) absorbed by empty exposure device
= power (watts) absorbed by empty exposure device
![]() = input power (watts)
= input power (watts)
![]() = output power (watts)
= output power (watts)
![]() = reflected power (watts)
= reflected power (watts)
Then place the sample in the exposure device and
determine ![]() , the power absorbed by both the sample and
exposure device, in a similar manner. Determine the total
absorbed power for the sample by taking the difference
between
, the power absorbed by both the sample and
exposure device, in a similar manner. Determine the total
absorbed power for the sample by taking the difference
between ![]() and
and ![]() . To determine the SAR, divide the total
absorbed power by the mass of the sample.
. To determine the SAR, divide the total
absorbed power by the mass of the sample.
The accuracy of these measurements can be improved by electronically measuring differential power: Use a differential amplifier or, for even more accuracy, connect the output of the power meters to an A-to-D converter and computer. Controlling temperature of the couplers and power meter heads also will improve the stability of the measurements.
The fields in a circular waveguide system can be quantified by differential-power determination of the SAR in the animal being exposed. To do this, subtract from the input power the power reflected out of the two transmitting and the two output ports. This requires five power meters.
Noninterfering Temperature-Probe Techniques--With the advent of RF noninterfering probes, a whole new field of SAR distribution determinations in realistic phantoms, cadavers, and live animals has become a reality. High resolution systems using thermistor detectors on high-resistance lead wires can reliably measure 0.01°C temperature changes in high-level RF fields. Lossy-line systems work best for frequencies above 100 MHz. For high fields below 100 MHz, line burnout may be a problem. Fiber-optic readouts are usable across the RF spectrum with minimal interference problems, but present instruments suffer from instability. Having the most sensitive technique possible is important if SAR distribution is to be measured. Less sensitive techniques require larger temperature rises, and more smearing of the SAR pattern will occur. Therefore, keep exposure time to a minimum to obtain minimum error from heat flow within the subject. Measure temperature rises in °C per minute; and for tissue and tissue-equivalent material, convert these data to SAR by
1ºC/min = 58.6 W/kg (Equation 7.7)
which is based on a specific heat of tissue of 0.84.
This measurement technique is the most accurate for assessing SAR distribution in phantoms and cadavers, allows temperature regulation to be measured in live animals, and is inexpensive. The major disadvantage is the time required to define the SAR in large or complex geometrical bodies. With the advent of systems with multiple temperature probes (Christensen and Volz, 1979), this problem has been largely reduced.
Calorimetric Techniques--Calorimetric techniques prove extremely useful in measuring whole-body SAR for animal phantoms and cadavers. Two techniques are now in practice: twin-well and dewar.
Twin-well calorimetry yields accurate results and requires little effort of the experimenter after initial setup and calibration. Difficulties include long run times for making one measurement (approximately 1 day for a large rat), complexity of multiple thermocouple readouts that make it difficult to detect failure in one or two thermocouples, and relatively high cost.
The dewar calorimeter technique is relatively inexpensive, is simple (calibration and operation anomalies are easily resolved), and requires a relatively short time for one reading (from 30 min for a mouse to 1 h for a rat). Accurate SAR determinations can be made with this technique, but they require experimenters to exercise extra precautions.
To check for heat loss during exposure, compare an SAR
measurement immediately after exposure with a measurement
that was delayed by a period equal to the exposure time. Water is usually used as the
heat transfer medium; use just enough to allow total
immersion of the cadaver. Adjust the water temperature to
approximately 0.5ºC below the ambient air temperature;
and after inserting the exposed animal, adjust the resultant
temperature by varying exposure time to obtain a temperature
rise of approximately 1ºC. This achieves maximum
stability in terms of calorimeter drift. When thermal
equilibrium is reached (i.e., when the change in T is less
than 0.01ºC during a 15-min period), measure the final
temperature, ![]() and use it to evaluate
and use it to evaluate ![]() for each cadaver:
for each cadaver:
where
![]() = rationalized temperature of the cadaver upon
insertion in the calorimeter
= rationalized temperature of the cadaver upon
insertion in the calorimeter
![]() = mass of the cadaver (kg)
= mass of the cadaver (kg)
![]() = specific heat of the cadaver (J·kg-1· K-1)
= specific heat of the cadaver (J·kg-1· K-1)
![]() = mass of water in calorimeter (kg)
= mass of water in calorimeter (kg)
![]() = specific heat of water (J·kg-1·K-1)
= specific heat of water (J·kg-1·K-1)
![]() = temperature of calorimeter just before insertion of
cadaver (ºC)
= temperature of calorimeter just before insertion of
cadaver (ºC)
![]() = final temperature of calorimeter (ºC)
= final temperature of calorimeter (ºC)
![]() = heat capacity of calorimeter (J·K-1)
= heat capacity of calorimeter (J·K-1)
The SAR in ![]() is determined by
is determined by
where
![]() = 3448 for a rat or mouse
= 3448 for a rat or mouse
![]() = 4185
= 4185
The readout of the calorimeter can be automated by direct computer readout; this also facilitates computations.
Thermographic Techniques--A scanning thermographic camera can be used to provide detailed SAR distribution in phantoms and cadavers in a short time. Suitable material to separate sections of the phantom or cadaver must be used, and readout after termination of exposure must be rapid. If the output of the thermographic camera is put into a computer, average SAR can be easily computed. Personnel at the University of Washington have developed this technique which is described in detail by Guy (1971a). The technique has proven valuable in assessing SAR distribution for laboratory animals and models of man. The procedure originally involved using a thin sheet of plastic to facilitate separating the halves of the phantom; thus the procedure was limited to symmetrical models exposed to a linearly polarized field (E-field parallel to the interface) to avoid interrupting any induced currents that would normally flow perpendicular to the median plane of separation (Guy, 1971a). For near-field measurements, however, the procedure was modified by replacing the plastic sheet with a silk screen, thus allowing easy separation without loss of electrical continuity (Guy et al., 1975a). The major disadvantage of this technique is the high cost of the required equipment.
Implantable Electric-Field Probes--Miniature electric-field probes with fiber-optics readout were developed by Bassen et al. (1977a). This system can be used to determine the E-field in a cadaver or phantom exposed to RF fields. The same equation used to determine E-fields from SAR (Chapter 2) can be used to determine SAR if the E-field is measured and the dielectric properties of the subject are known. The advantages of this technique include instant readout (allowing SAR distribution to be determined by scanning techniques) and accurate measurements in low-level fields (this technique being at least 10 times as sensitive as any technique previously discussed). The primary problems include probe rigidity (requiring straight insertion into the subject) and frequency-range limitation. The use of orthogonal probes simplifies measurements, but single-axis probes can be used. Subminiature probes (2-mm diameter) are under development. A review of implantable E-field probes is given by Bassen et al. (1983).
Use of Phantoms to Measure SAR--SAR can be measured in animal cadavers; but measuring in phantoms is sometimes more convenient, more reproducible, and almost as accurate. A mold of RF-transparent foam material can be made in the shape of the phantom to be investigated, and the mold filled with a tissue equivalent material. Researchers in the Department of Rehabilitation Medicine, University of Washington School of Medicine, have developed materials to simulate muscle, brain, fat, and bone for various frequency ranges (Guy, 1971a; Chou et al., 1984).
Tables 7.1-7.4 summarize the directions for preparing the simulated tissue material, Tables 7.5-7.7 list the composition and properties, and Table 7.8 gives sources for the ingredients. Figures 7.2 and 7.3 show the electrical properties as a function of frequency and temperature.
Tables 7.9-7.11 give directions for other tissue-equivalent materials, developed at the University of Ottawa (Hartsgrove and Kraszewski, 1984). The material in Table 7.9 includes hydroxyethylcellulose to make preparation easier and to provide stability of the material over longer periods of time. Table 7.10 describes a more liquid material that has electrical properties equivalent to tissue.
Knowledge of thermal properties of tissue-equivalent materials is useful in determining how fast thermal diffusion will cause heating patterns to change. Leonard et al. (1984) determined the thermal properties of some common tissue-equivalent materials. Tables 7.12 and 7.13 list formulas for other muscle- and fat-equivalent materials for the 10-50-MHz frequency range, also the thermal and electrical properties. These materials, in which barium titanate is used instead of aluminum powder, were designed to have a lower thermal diffusivity to minimize smearing of the heating patterns.
Table 7.1.
Directions For Preparing Simulated Muscle Material For 13.56-100 MHz (Guy, 1971a; Chou et al., 1984)
Table 7.2.
Directions For Preparing Simulated Muscle Material For 200-2450 MHz (Guy, 1971a; Chou et al., 1984)
Table 7.3.
Directions For Preparing Simulated Brain Material For 915 AND 2450 MHz (Guy, 1971a; Chou et al., 1984)
Table 7.4.
Directions For Preparing Simulated Fat And Bone Materials For 915 AND 2450 MHz (Guy, 1971a; Chou et al.,
1984)
Table 7.5.
Composition and Properties Of Simulated Brain,
Fat, And Bone Tissue At Microwave Frequencies(Guy, 1971a)
Table 7.6.
Composition and Electrical Properties Of Simulated Muscle For 13.56-2450 MHz (Chou et al., 1984)
Table 7.7.
Electrical Properties Of Simulated Muscle For 13.56-2450 MHz At Three Temperatures (Chou et al., 1984)
Table 7.8.
Some Sources* Of Materials Used To Construct
Phantom Models (Guy, 1971a; Chou et al., 1984)
Figure 7.2
Relative permittivity of simulated muscle
tissue versus frequency for three temperatures (Chou et al.,1984).
Figure 7.3
Electrical conductivity of simulated muscle
tissue versus frequency for three temperatures (Chou et al., 1984).
Table 7.9.
Composition And Electrical Properties Of Tissue-Equivalent Materials (Hartsgrove and Kraszewski, 1984)
Table 7.10.
Composition And Electrical Properties Of A Liquid That Has The Electrical Properties Of Tissue (Hartsgrove and Kraszewski, 1984)
Table 7.11.
Directions For Preparing The Tissue-Equivalent Materials Described In Tables 7.9 And 7.10 (Hartsgrove and Kraszewski, 1984)
Table 7.12.
Simulated Phantom Material Using Barium Nitrate
Table 7.13.
Simulated Fat Material

Go to Chapter 7.2.6.
Return to Table of Contents.
Electrical Properties Of Simulated Muscle For 13.56-2450 MHz At Three Temperatures (Chou et al., 1984)
Some Sources* Of Materials Used To Construct Phantom Models (Guy, 1971a; Chou et al., 1984)
Relative permittivity of simulated muscle tissue versus frequency for three temperatures (Chou et al.,1984).
Electrical conductivity of simulated muscle tissue versus frequency for three temperatures (Chou et al., 1984).
Composition And Electrical Properties Of Tissue-Equivalent Materials (Hartsgrove and Kraszewski, 1984)
Composition And Electrical Properties Of A Liquid That Has The Electrical Properties Of Tissue (Hartsgrove and Kraszewski, 1984)
Directions For Preparing The Tissue-Equivalent Materials Described In Tables 7.9 And 7.10 (Hartsgrove and Kraszewski, 1984)
Simulated Phantom Material Using Barium Nitrate
Simulated Fat Material
Last modified: June 24, 1997
© October 1986, USAF School of Aerospace Medicine,
Aerospace Medical Division (AFSC), Brooks Air Force Base,
TX 78235-5301